Intuitive Software
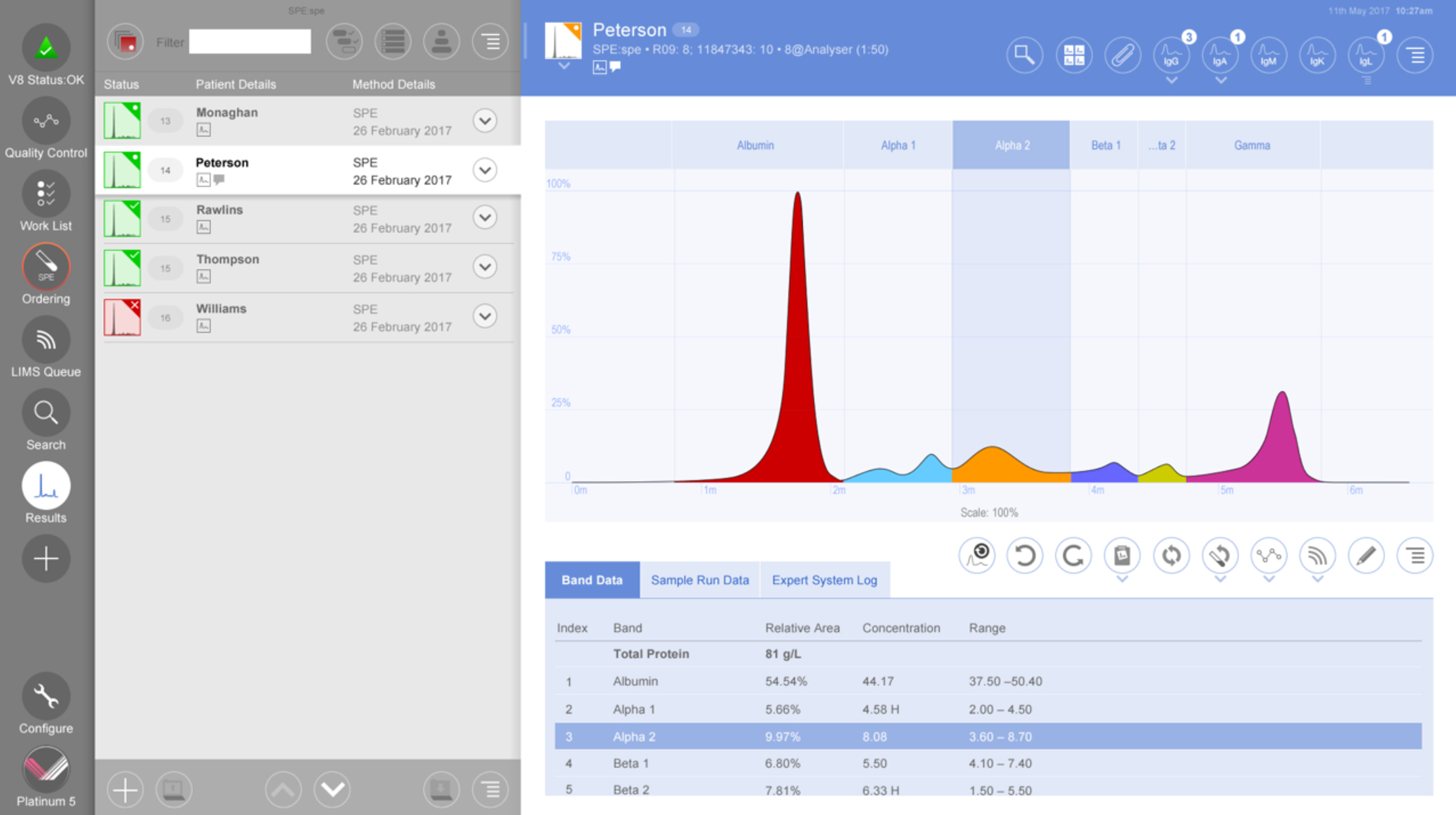
• Useful touch screen operation
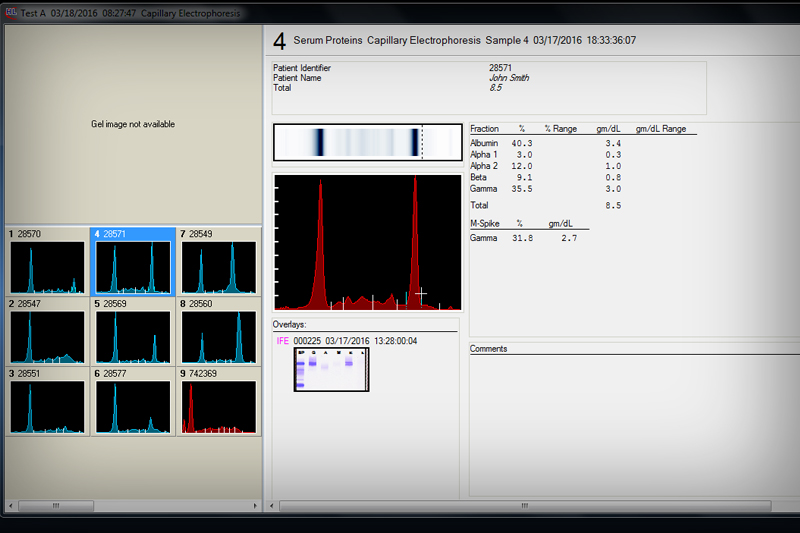
• Automates identification of normal samples
• Easily overlay traces and monitor changes
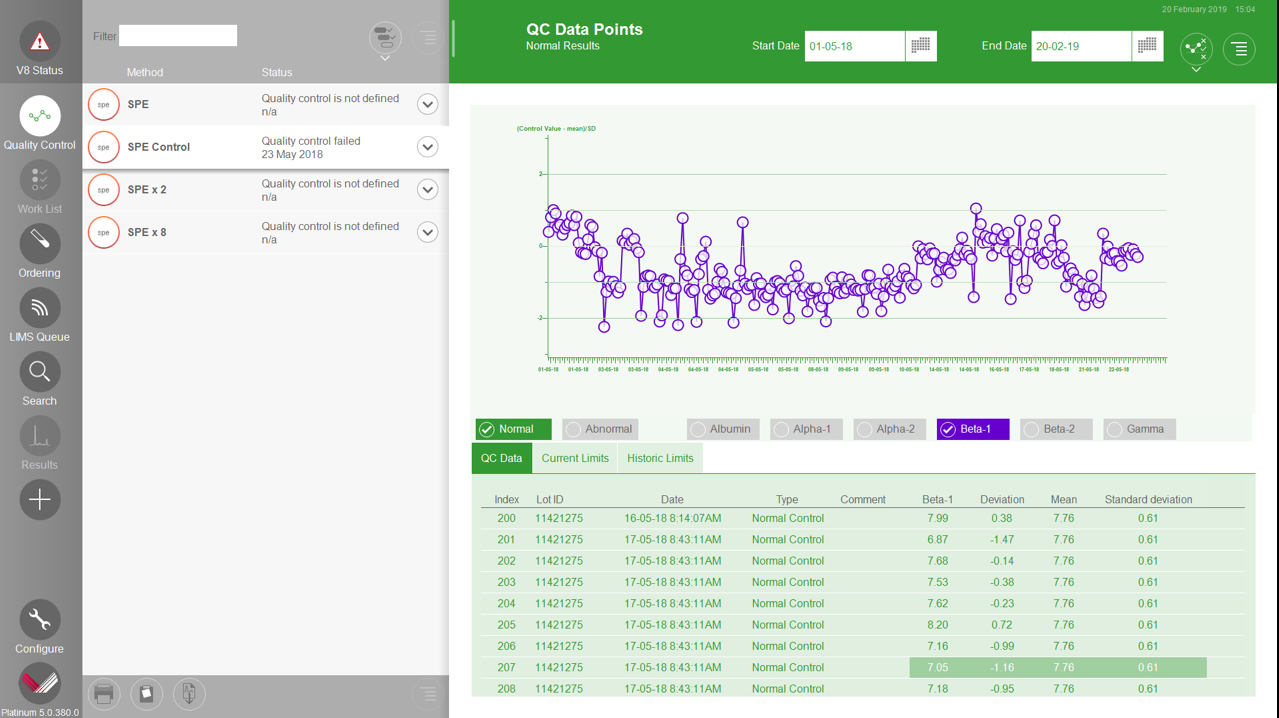
Platinum 6 Improved QC Suite
• User-defined Levey-Jennings & Westgard rules
• Live feedback of QC status
• Easy identification of QC failures
• Analyze individual band plots
• Live feedback of QC status
• Easy identification of QC failures
• Analyze individual band plots